NURFARAH YASMIN BINTI MAT SAAD's Reflection
Assalammualaikum and Hi everyone.
So for the last 3 months, I've been doing internship at Duopharma Biotech Berhad Klang. It is a pharmaceutical company that produces tablet and powder of medicine product and also Vitamin. The most common or familiar product that we might heard is Flavettes, Champ, Proviton, Uphamol, and Eye glo. It quite excited for me to enter this pharmaceutical company and learn something new since the scope study of mine is a bit difference with the pharmaceutical career. However i was placed at Quality Control department where i learn and do many chemical lab works. So there are some of equipment and apparatus that familiar for me since I've learn analytical and physical chemistry during my degree studies.
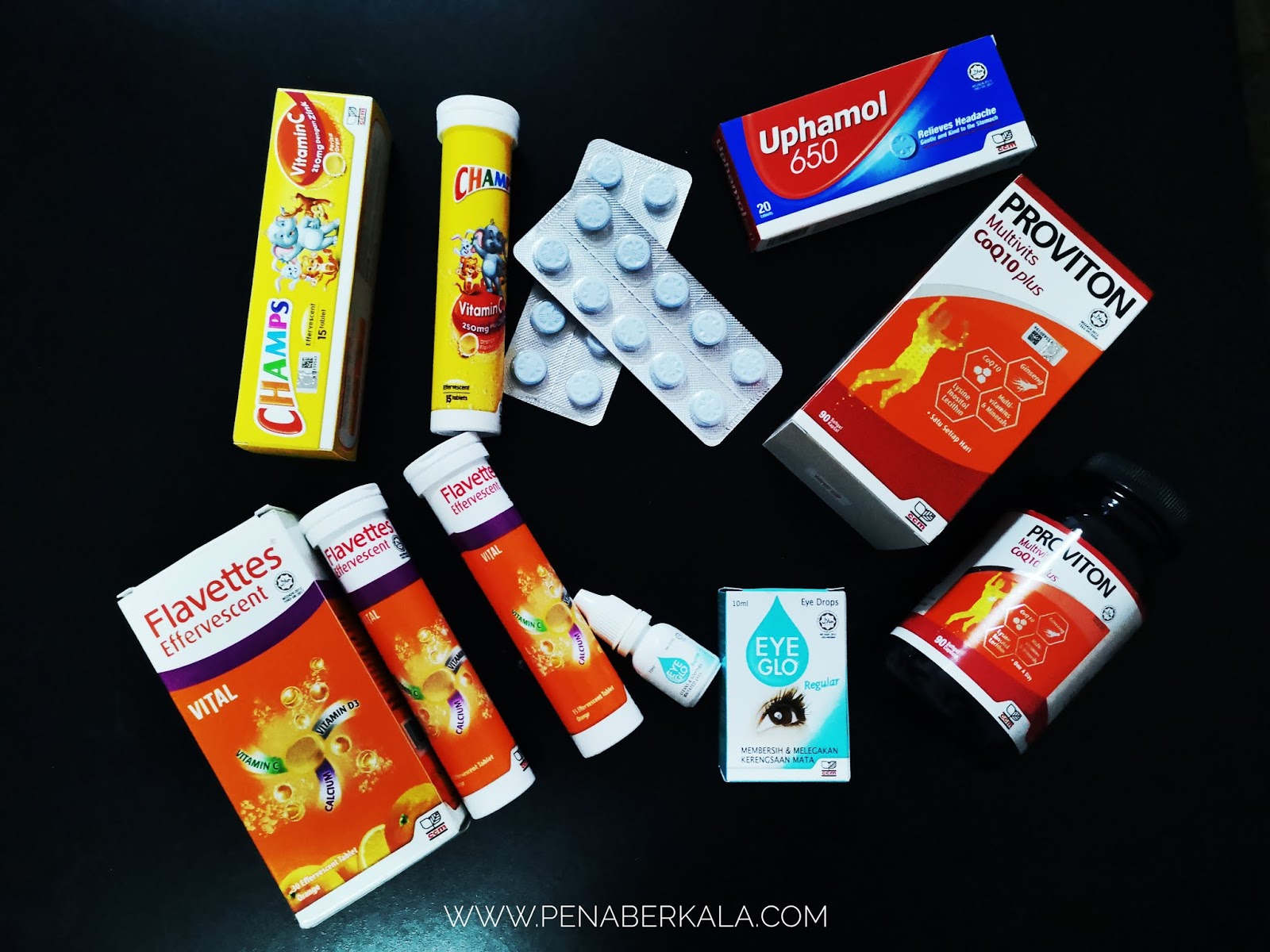
Quality Control department is a place where we need to test all the raw materials, in process and finished product before we proceed to production and packaging part. During my internship, i've learn to test the tablet and powder of product such as Metazine, Glucoxit, Zhubee Vitamin C, Royal Jelly Vitamin C, Betawin, Acugesic, Betamox, and so on. There will be 3-4 testing and we called it as physical test for the all the tablets. Firstly, there will be the disintegration test where we can see the duration for the tablets to dissolve in the water at 37 of degree Celsius ( same as stomach temperature). Second, there will be the Frability part where we can test the durability of the tablets within 100 falling. Each of the test for each type of tablet need to take 20 tablets. Third, I also do the hardness, diameter and thickness of the tablets. Every 20 tablets done will proceed to the report. Other than that, each type of batch need to be weighed for 20 tablets.
Next, I also learned and was assigned to the cyanoguanidine testing where we need to see any purities in the tablets within the acceptance limit range. I learned to use HPLC which High Performance Liquid Chromatogram to reviewed and see the peak of the result after its injection of the solution. After that, I also learn how to integrate the peak shows in the results same as standard results. There will be 2 types of quality control where the powder product will be test in IPQC which is In-Process Quality Control and tablets product will be test in FPQC which is Finished-Product Quality Control. All of these process need to be followed by the SOP that produce by senior executive and manager. This is because there will be an update of the procedure. In the other hand, I also learn to do the Assay testing for the tablets. For Assay, each batch of tablet need to be tested duplicate. For powder there will be top, middle and bottom, so there's no need to do duplicate for the product. This is because top, middle and bottom are refer to the powder in the column during the production. They will take each place in the column and divide into three different packaging to test.
It's been an amazing and interesting experience for me during this 3 months of Internship in Duopharma Biotech. All colleague and supervisor are friendly and sporting. They teach and guide me slowly and patiently. To be honest, the environment in this place is very very positive and good. Even though some of the staffs are working for long time in the company, but they are all friendly and easy to talk with. I feel so comfortable to ask something or discussed about something that i do not know and blurred about. However, the work scope is a bit pressure since all the report need to be submit within the day after it was assigned for us. So everyday will be different task to do, and yesterday report need to be submit by the next day. We have to ready mental and physical to finished all the work., but mostly the supervisor will give a few day to complete the report. That's all my sharing during my internship.
Thank you for reading! hope u enjoyed hehe!
